August 8, 2025 (August 7, New York time) — Professor Weihong Song, Fellow of the Canadian Academy of Health Sciences, Director of Oujiang Laboratory, Academic Vice President of Wenzhou Medical University, and Director of the International Center for Alzheimer's Disease Research and Prevention, was invited to publish an article titled “Improving Alzheimer's Disease Immunotherapy” . The article provides an in-depth review of a novel monoclonal antibody brain delivery method that demonstrates enhanced efficacy and safety in Alzheimer's disease animal models, exploring and outlining approaches to improve immunotherapy for Alzheimer's disease.

“Engineered” Antibodies: Precise, Efficient Brain Penetration
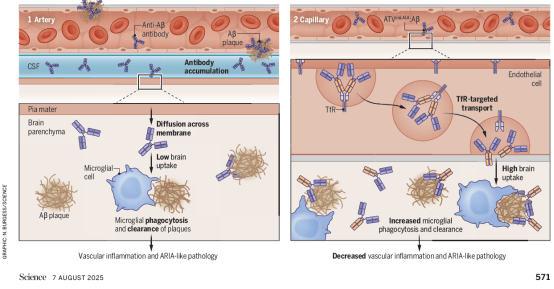
Alzheimer's disease is the most prevalent neurodegenerative brain disorder, commonly known as senile dementia. Its hallmark pathology involves abnormal accumulation of amyloid-β protein (Aβ) forming plaques within the brain. In recent years, AD immunotherapies targeting the clearance of Aβ and related pathological molecules have emerged as a research and clinical focus. Currently, three monoclonal antibody drugs targeting Aβ clearance—Aducanumab, Lecanemab, and Donanemab—have received U.S. Food and Drug Administration (FDA) approval for intravenous administration in treating early-stage AD. However, their clinical application faces two major challenges: First, inefficient brain penetration, as the blood-brain barrier (BBB) impedes antibody entry into the brain; Second, frequent adverse effects, particularly the occurrence of “Amyloid-related imaging abnormalities” (ARIA), characterized by cerebral edema (ARIA-E) and microhemorrhages (ARIA-H). These side effects are more prevalent in individuals carrying high-risk AD genes such as the apolipoprotein E ε4 (ApoE ε4) allele.
Traditional antibody drugs enter brain tissue indirectly via cerebrospinal fluid through a compromised BBB. However, this method is inefficient for reaching the brain parenchyma, and circulating antibodies tend to accumulate around cerebral blood vessels. There, they bind to Aβ deposits on the vascular walls, triggering inflammatory responses and microbleeds.
This article details and reviews the research findings published concurrently in Science by Pizzo et al., titled “Transferrin receptor-targeted anti-amyloid antibody enhances brain delivery and mitigates ARIA.” The Zuchero research team employed a “piggybacking” strategy, utilizing the transferrin receptor on the surface of cerebral vascular endothelial cells as a “channel.” This allows antibodies to actively traverse the blood-brain barrier via natural receptor transport mechanisms, precisely entering brain parenchymal tissue while avoiding sensitive vascular areas. This approach enhances therapeutic targeting and safety.
ATVcisLALA:Aβ: A Potential Next-Generation Modified Antibody
To mitigate potential side effects of conventional antibodies, researchers implemented a “dual modification” to the antibody structure: First, the introduction of the “ATV” (Antibody Transport Vehicle) structural unit enables the antibody to recognize and utilize the transferrin receptor pathway to actively cross the blood-brain barrier while minimizing contact with Aβ on the vascular wall. Second, the lysine (L) residues at positions 234-235 in the antibody's Fc region were mutated to alanine (A) (the “LALA” mutation, Leu234Ala/Leu235Ala). This reduces its binding affinity to receptors (FcγR) that specifically recognize the Fc segment of immunoglobulin G (IgG) on other cells, thereby minimizing immune system side effects, preventing damage to blood cells, and enhancing safety. This ultimately yielded a novel antibody termed ATVcisLALA:Aβ. Results demonstrated a 5–8-fold increase in brain antibody concentrations in AD mouse models, significantly reducing vascular inflammation and ARIA-like lesions associated with conventional antibodies. Crucially, it retains the ability to activate microglia for effective clearance of intracerebral Aβ deposits, balancing efficacy with safety.
Long-Term Potential: Beyond Aβ
The review article highlights that, in addition to Aβ, Alzheimer's disease is also characterized by abnormal phosphorylation and aggregation of tau protein within neurons. Several Tau-targeting antibodies are currently in development. Leveraging the ATV platform could potentially yield superior therapeutic outcomes in the future. Moreover, the ATV platform holds promise for application in other central nervous system disorders such as Parkinson's disease and Huntington's disease, and may even address rare lysosomal storage disorders. By enabling precise delivery of therapeutic enzymes or proteins into brain tissue, it could overcome longstanding barriers to drug administration.
A Forward-Looking Perspective on Alzheimer's Disease Prevention and Treatment
The commentary article highlights that this research not only provides a novel framework for anti-Aβ immunotherapy but also challenges the traditional view that ARIA is inevitable. As an expert with nearly 40 years of dedicated research in Alzheimer's disease, Song Weihong emphasizes that the core of effective prevention and treatment lies in identifying precise biological markers for early diagnosis, uncovering the pathogenic mechanisms leading to dementia, and thereby developing effective preventive measures and therapeutic drugs. Current advancements in immunotherapy focus on precisely controlling the antibody's “functional structural modules” and “brain entry pathways.” This novel monoclonal antibody delivery strategy not only enhances therapeutic efficacy but also holds promise for reducing risks, paving a new path for future AD immunotherapy.
Dubbed the “killer of memory,” Alzheimer's disease currently affects over 10 million patients in China, with projections exceeding 40 million by 2050. Any therapeutic strategy that enhances efficacy while reducing risks could bring hope to millions of families. Chinese scientists are not only making significant contributions in cutting-edge research but also advancing global understanding of AD mechanisms and treatments. We anticipate that these “engineered antibodies” will transition from the laboratory to clinical practice, delivering truly breakthrough changes for AD patients.
Manuscript URL:
https://www.science.org/doi/10.1126/science.adz8959