On October 10, the research group led by Zhifeng Huang and Lintao Song from Academician Xiaokun Li's team at Oujiang Laboratory published a significant finding in Cell Metabolism (IF=27.7), a top-tier international journal in the field of metabolism: “Hepatic FXR-FGF4 is required for bile acid homeostasis via a FGFR4-LRH-1 signal node under cholestatic stress.”

This study identified FGF4 in the liver as a direct target gene of FXR, which downregulates key bile acid synthesis enzymes CYP7A1 and CYP8B1 through paracrine signaling. This liver-centric pathway functions as a primary checkpoint for intrahepatic bile acid levels. Together with FGF15/19 secreted by the intestine, it forms a hepaticointestinal regulatory circuit that precisely modulates bile acid homeostasis to counteract cholestasis and its associated liver damage. This work provides compelling evidence supporting the FXR-FGF signaling axis theory of bile acid regulation over the past two decades, offering novel therapeutic insights and strategies for cholestasis and related disorders. It represents another significant scientific discovery by the Huang Zhifeng laboratory, following last year's finding that a single central administration of FGF4 produces sustained glucose control effects lasting over 7 weeks (Cell Metabolism, 2023, 35(6):1022-1037).
Bile acids are essential for the digestion and absorption of fat-soluble nutrients while also serving as signaling molecules regulating metabolic and energy homeostasis. Disruption of bile acid homeostasis or related signaling pathways can lead to cholestasis, liver injury, and metabolic disorders, potentially progressing to hepatocellular carcinoma in severe cases. Bile acid homeostasis is primarily maintained through negative feedback mechanisms and spatiotemporally regulated enterohepatic circulation. Regulation of bile acid synthesis relies heavily on the activation of farnesoid X receptor (FXR) in the liver and intestine, which senses bile acid levels and controls gene transcription related to bile acid synthesis, conversion, transport, and signaling.
Previous studies indicate that activated FXR promotes hepatic transcription of SHP and MAFG, as well as ileal transcription of fibroblast growth factor 15/19 (FGF15/19), in response to postprandial increases in intestinal bile acids. Intestinally secreted FGF15/19 selectively binds and activates the hepatocyte fibroblast growth factor receptor 4 (FGFR4)-β-klotho (KLB) complex, inhibiting transcription of key bile acid synthase genes Cyp7a1 and Cyp8b1 via an as-yet-unresolved signaling pathway. This endocrine pathway, coupled with physiological responses to food digestion, blocks new bile acid synthesis postprandially. This represents a necessary physiological requirement to prevent the potential toxicity of excessive bile acids to the liver and intestinal systems. Because the ileum is separated from the liver—the primary organ for bile acid synthesis—the ileal FGF15/19 pathway functions later than the liver's role in early postprandial bile acid synthesis. This spatiotemporal constraint raises a critical question: How is hepatic bile acid synthesis and total bile acid content regulated in the liver when the endocrine FGF15/19 signaling pathway is not yet active?
To address this question, the research team generated knockout mice with endocrine FGF15 deficiency. Results revealed that in the absence of Fgf15, FXR activation still maintained suppression of Cyp8b1 expression but failed to inhibit Cyp7a1. Interestingly, this inhibitory effect was completely lost in the livers of mice lacking the corresponding receptor Fgfr4. Consequently, the research team proposed a key hypothesis: another FGF family member regulated by FXR exists in the liver, directly activating hepatic FGFR4 via autocrine or paracrine pathways to suppress bile acid synthesis, particularly by inhibiting the Cyp8b1 pathway.
To validate this hypothesis, the team systematically screened FGF family members to identify those causing differential FXR effects. They discovered that hepatic FGF4 serves as another direct target gene of FXR. Acting as a signaling mediator for hepatic FXR, FGF4 inhibits the transcription of both Cyp7a1 and Cyp8b1—with more pronounced suppression of Cyp8b1—thereby reducing hepatic bile acid synthesis and ameliorating cholestasis. Further mechanistic studies revealed that this liver-centric FXR-FGF4 interaction is mediated through a previously unreported FGFR4-LRH-1 signaling pathway: upon FGF4 activation of FGFR4, the cytoplasmic nuclear receptor LRH-1 is recruited to the FGFR4 kinase domain,
To validate this hypothesis, the research team systematically screened and analyzed FGF family members responsible for FXR's differential effects, identifying hepatic FGF4 as another direct target gene of FXR. Acting as a signaling mediator for hepatic FXR, FGF4 inhibits the transcription of Cyp7a1 and Cyp8b1—with a more pronounced effect on Cyp8b1—thereby suppressing hepatic bile acid synthesis and alleviating cholestasis. Further mechanistic studies revealed that this liver-centric FXR-FGF4 interaction is mediated through a previously unreported FGFR4-LRH-1 signaling pathway: Upon FGF4 activation of FGFR4, the cytoplasmic nuclear receptor LRH-1 is recruited to the FGFR4 kinase domain. This leads to LRH-1 inactivation via phosphorylation, thereby eliminating its targeting effect on the Cyp7a1 and Cyp8b1 gene promoters. Crucially, the phenomenon observed in mice was fully validated in clinical patients, where cholestasis patients exhibited significantly reduced hepatic FXR and FGF4 expression levels and impaired signaling axes compared to healthy individuals.
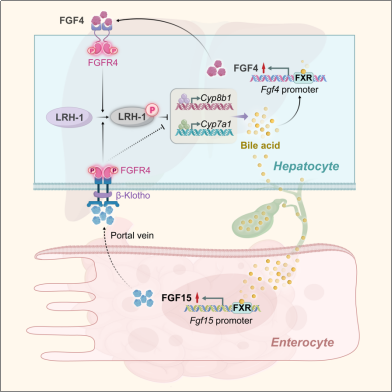
In summary, this study identifies hepatic FGF4 as a direct transcriptional target of FXR and a liver-centric feedback inhibitor of bile acid synthesis upstream of the peripheral FGF15/19 pathway. Precise spatiotemporal control of bile acid synthesis is crucial for maintaining bile acid homeostasis in response to diverse physiological demands and pathological challenges. The elucidation of this hepatic FXR-FGF4 to FGFR4-LRH-1 pathway clarifies and expands the mechanisms and patterns of spatiotemporal regulation. These findings provide novel mechanistic insights into hepatic bile acid synthesis and the regulation of enterohepatic or systemic bile acid homeostasis, opening new avenues for designing therapeutic strategies for cholestasis and related diseases.
Manuscript URL:
https://www.cell.com/cell-metabolism/abstract/S1550-4131(24)00372-3